BrdU: Re-Discover the Lab Favorite for Cell Proliferation Analysis
What is BrdU? - chemical structure and use in cell proliferation assays
Incorporation of the thymidine analog 5′-bromo-2′-deoxyuridine (BrdU) has been established as a popular assay for determining cell proliferation rates in a wide variety of species, ranging from plants to mammalian cells (Cecchini et al. 2012, Nagar et al. 2002). One common assay set-up is to supplement the culture media of growing cells with BrdU. By doing so, replicating cells, during the S phase of the cell cycle, incorporate BrdU instead of thymidine into newly synthesized DNA.
BrdU is characterized by a very similar chemical structure to thymidine. The structural difference between BrdU and thymidine is illustrated in Figure 1; in the case of BrdU, the 5-methyl group (shown in blue) of thymidine has been substituted by bromine (highlighted in green) (Kolb et al. 1999, Sivakumar et al. 2004).
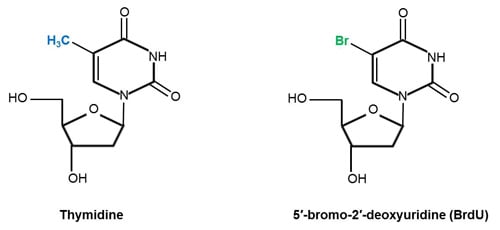
Fig.1. Chemical structures of the DNA nucleoside thymidine and its analog BrdU. Structures adapted from Liboska et al. (2012) (BrdU) and Young et al. (1969) (thymidine).
Alternatively to bromine, thymidine’s 5-methyl group can be replaced by other halogens such as chlorine (5′-chloro-2′-deoxyuridine, CldU) and iodine (5′-iodo-2′-deoxyuridine, IdU) (Sivakumar et al. 2004).
Detection of incorporated BrdU with anti-BrdU antibodies
Incorporated BrdU can be readily detected with anti-BrdU antibodies, such as the Mouse Anti-BrdU Antibody, clone Bu20a (MCA2483) and Rabbit Anti-BrdU Antibody (AHP2405). However, for successful staining it is important to include a DNA denaturing step to allow the antibody access to the incorporated BrdU. Acids, such as hydrochloric acid, are frequently used for this purpose; although treatments with copper ions, heat, nucleases, and sodium hydroxide have also been reported (Liboska et al. 2012, Kennedy et al. 2000).
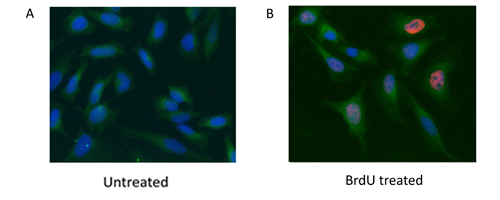
Fig. 2. HeLa cells were treated with 10 μg BrdU for 1 hr (B) or left untreated (A). Cells were stained with Mouse Anti-BrdU Antibody, clone Bu20a (MCA2483) (red) and Rabbit Anti-GAPDH Antibody (AHP1628) (green). PureBlu™ DAPI Nuclear Staining Dye (1351303) was used as a nuclear counterstain.
Explore the BrdU Labeling and Staining Protocols Read “5 Top Tips to Control your BrdU Labeling Experiments"
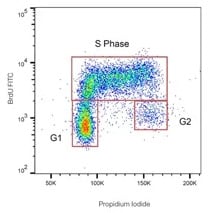
Detection of BrdU is commonly performed in combination with total DNA staining. When performing cell cycle analysis, propidium iodide (PI) tends to be the DNA stain of choice (Figure 3).
Fig. 3. BrdU-labeled human lymphoma cells stained with Mouse Anti-BrdU Antibody, clone BU20a, (MCA2483) at a 1/100 dilution overnight at 4°C. As secondary antibody FITC conjugated goat anti-mouse IgG (H/L) (STAR117F) was used at a 1/50 dilution. Total DNA staining was performed with ReadiDrop™ Propidium Iodide (1351101).
View BrdU Staining of Cells for Cell Cycle Analysis Protocols
BrdU synonyms:
5-bromo-2'-deoxyuridine, 5-bromodeoxyuridine, BrdUrd, bromodeoxyuridine, broxuridine, BUdR (PubChem CID: 6035)
References
- Cecchini MJ et al. (2012). Analysis of cell cycle position in mammalian cells. J Vis Exp 59, 3491.
- Liboska et al. (2012). Most anti-BrdU antibodies react with 2'-deoxy-5-ethynyluridine -- the method for the effective suppression of this cross-reactivity. PLOS One, 7 e51679.
- Kennedy BK et al. (2000). Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 15, 2855-2868.
- Kolb B et al. (1999). Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neuro 19, 2337-2346.
- Liboska R et al. (2012). Most anti-BrdU antibodies react with 2′-deoxy-5′-ethynyluridine – the method for the effective suppression of this cross-reactivity. PLoS One 7, e51679.
- Nagar S et al. (2002). Host DNA replication is induced by geminivirus infection of differentiated plant cells. Plant Cell 14, 2995-3007.
- National Center for Biotechnology Information. PubChem Compound Database; CID=6035, https://pubchem.ncbi.nlm.nih.gov/compound/6035, accessed May 15, 2017.
- Sivakumar S et al. (2004). In vivo labeling of fission yeast DNA with thymidine and thymidine analogs. Methods 33, 213-219.
- Young DW et al. (1969). The crystal and molecular structure of thymidine. Aca Cryst B25, 1423-1432.