Mini-review: An Overview of B Cells - from Discovery to Therapy
In the past two decades, significant advances have been made in B cell biology. These critical immune cells remain an active area of research particularly because disruption of B cell development or function results in a number of autoimmune diseases and malignancies. In addition to producing antibodies, B cells are professional antigen presenting cells that can present antigens to T cells to generate effective immune responses. B cells are however, a heterogeneous population of cells at different stages of maturation along the lineage, each with unique functional properties. This mini-review provides a brief history of the discovery of B cells, as well as describes the characteristics of each B cell lineage and the processes of B cell development, maturation and activation. Finally, we highlight the application of B cell biology in the development of novel therapeutics for the treatment of B cell mediated diseases.
A brief history of the discovery of B cells
B cells are an integral part of the adaptive immune response. They represent a distinct lineage, with separate and unique functions from T cells. In addition to producing antibodies, they perform critical immune functions such as generating immunological memory, antigen presentation and regulatory cytokine production. Our current understanding of B cell biology was initiated in 1965 with a landmark study by Max Cooper and Robert Good. Using chicken as an experimental model system, they showed that cells that develop in the bursa of Fabricus (equivalent to the bone marrow in mouse and human) are responsible for antibody production (B cells), whereas cells that develop in the thymus are responsible for delayed-type hypersensitivity responses (T cells) (Cooper et al. 1965). This finding defined separate B and T cell lineages in adaptive immunity.
However, the first indication of the existence of B cells was in 1890 when Emil von Behring and Shibasaburo Kitasato discovered that circulating “antitoxins” (now known to be antibodies) were important in immunity to diphtheria and tetanus (von Behring and Kitasato 1890). Paul Ehrlich later proposed that cells with pre-formed antibody receptors (now known to be B cell receptors) were the possible producers of these “antitoxins” (Ehrlich 1967).
In the late 1940s, the cellular source of antibodies (B cells) was identified. It was shown that plasma cell development correlated with antibody responses after immunization (Fagraeus 1948). Following this discovery were two competing views of antibody formation, the natural selection theory (Jerne 1955) and the clonal selection theory (Burnet 1959). Experimental evidence supporting the clonal theory demonstrated that each antibody producing cell makes one type of antibody, and is stimulated by its cognate antigen to produce and secrete more of the same kind of antibody (Nossal and Lederberg 1958). This theory has been modified over the years but still serves as a guiding principle of adaptive immunity.
In the mid-1960s to early 1970s, advances were made in the characterization of B cells using animal models, clinical evaluation of patients with immune deficiency diseases and the innovation of cell surface molecule characterization (LeBien and Tedder 2008). In 1968, mouse transplant studies showed that cells derived from the bone marrow mediated antibody responses (Mitchell and Miller 1968).
A developmental link between B cells and antibody production was established with studies showing that surface immunoglobulin (Ig) expression could be used as a marker of normal and leukemic B cells (Froland et al. 1971; Le Bien and Tedder 2008). Around this time, immunologists also discovered somatic mutations in the antibody light chain variable region (Weigert et al. 1970). In 1974, B cells were shown to originate from the fetal liver and bone marrow in mice (Cooper 2015), and their precursors, pre-B cells, were later identified in the fetal liver and bone marrow of mice (Raff et al. 1976) and humans (Gathings et al. 1977).
Up until 1980, the molecular composition of the cell surface of B cells was largely uncharacterized, and the B cell surface was known to only consist of bound Ig, complement receptors and Fc receptors (LeBien and Tedder 2008). This changed with the introduction of monoclonal antibody technology by Cesar Milstein in 1975 (see Table 1 for common B cell specific markers). The first B cell specific molecule described was termed B1 and is now known as CD20 (Stashenko et al. 1980).
In 2002, a new subset of B cells with regulatory functions was identified (Mizoguchi et al. 2002). These B regulatory cells were shown to produce the anti-inflammatory cytokine IL-10, and suppress inflammatory responses in experimental autoimmune encephalomyelitis, collagen-induced arthritis and autoimmune colitis (Fillatreau et al. 2002; Mauri et al. 2003). These cells have also been shown to directly inhibit T cell proliferation in murine models (Wei et al. 2005). The phenotypic nature of B regulatory cells in mouse is currently being debated and there appears to be two regulatory B cell lineages in mice. B cells with regulatory function have also been identified in humans (Tobòn et al. 2013).
The year 2015 marked the 50th anniversary of the landmark study that identified B cells as a separate lineage of the adaptive immune system (Cooper et al. 1965). During this time several advancements in B cell biology have been made and this mini-review summarizes the current understanding of B cell biology.
Table 1. Cell surface CD markers preferentially expressed by B cells
Name |
Function |
B Cell Subtype Expression |
|---|---|---|
|
Regulates intracellular B cell signaling by amplifying Src-family kinase activity |
Expressed on all B-lineage cells; Pan B cell marker |
|
|
Functions as a membrane embedded Ca2+ channel |
Mature B cells |
|
|
C3d and Epstein-Barr virus receptor that interacts with CD19 to induce B cell inflammatory responses |
Mature B cells |
|
|
Functions as a mammalian lectin for α2,6-linked sialic acid that regulated follicular B cell survival |
Mature B cells |
|
|
Low affinity IgE receptor that influences IgE production |
Activated B cells |
|
|
Function is still unknown |
Expressed on all B-lineage cells; Pan B cell marker |
|
|
Critical survival factor for GC B cells; ligand for CD154 expressed by T cells |
B cells |
|
|
Negative regulator of signal transduction; B cell ligand for CD100 (Semaphorin 3D) |
Expressed on all B-lineage cells; Pan B cell marker |
|
|
Contain highly conserved motifs in their cytoplasmic domains for tyrosine phosphorylation and Src family kinase docking to initiate B cell activation |
Surface Ig+ B cells |
Adapted from LeBien and Tedder 2008. Ig, immunoglobulin; GC, germinal center
B cell development and maturation
B cell development begins with the migration of multipotent progenitor cells (MPPs) first into the fetal liver and then into the bone marrow. MPPs then differentiate into the common lymphoid precursor (CLP) that ultimately produces the common lymphoid 2 progenitor (LCA-2) that is responsible for the B cell lineage (Tobòn et al. 2013). LCA-2 receives signals from stromal bone marrow cells to induce the development of B cells. These signals include interleukin (IL)-7 and Fms-like tyrosine kinase 3 ligand (Flt3-L) as well as the action of transcription factors such as PU.1, IKAROS (IKAROS family zinc finger 1), E2A, EBF (early B cell factor 1), PAX5 (paired box gene 5) and IRF8 (interferon regulatory factor 8) (Tobòn et al. 2013; Melchers 2015).
A critical step in B cell development is the generation of the B cell receptor (BCR). Because mature B cells express only one type of BCR for a specific antigen (clonal BCR distribution), a large repertoire of BCRs are required to ensure the selectivity and specificity of the adaptive immune response. This is accomplished through the rearrangement of Ig gene segments and nucleotide insertion mechanisms at gene segment junctions (Komori et al. 1993; Tonegawa 1983; Alt et al. 1984). The heavy and light chain loci of Igs are composed of a series of V (variable) gene elements, followed by several D (diversity) segments (only on heavy chain gene), J (joining) segments and C (constant region) exons (Tobòn et al. 2013).
Nucleotide insertion of VDJ gene rearrangement is mediated by terminal deoxynucleotidyl transferase (TdT) and recombinase activating genes 1 and 2 (RAG1/2), which are induced in lymphoid progenitors in response to cytokines secreted by stromal bone marrow cells. B cell progenitor cells initially undergo D-J gene segment joining on the heavy chain chromosome (D-JH) to become early pro-B cells. Subsequent joining of a V segment to the D-JH gene segment completes the late pro-B cell stage.
The pre-B cell is then formed following successful VHDJH recombination, the resulting heavy chain pairs with a surrogate light chain (SLC), comprised of two distinct proteins λ5 and VpreB, to form the pre-BCR. The initially formed heavy chain utilizes the JH-proximal µ constant region. The pre-BCR complex includes the signaling components Ig-α and Ig-β and is expressed on the cell surface of pre-B cells. Signaling through the pre-BCR is crucial for continued B cell development, and leads to reduced RAG1/2 protein levels and an increase in proliferation to form large pre-B cells (Kitamura et al. 1992).
Eventually, large pre-B cells stop proliferating and RAG1/2 proteins are re-expressed to induce light chain rearrangement, marking the small pre-B cell stage. Successful light chain rearrangement at either the Ig kappa or lambda light chain foci leads to the expression of IgM as a complete BCR on immature B cells.
Notably, although B cell development has primarily been studied in mice, human B cell development was shown to be similar (Naradikian et al. 2014; Nunez et al. 1996). One major difference however is that murine pro- and pre-B cells depend on IL-7 for survival and differentiation, whereas human B cell development is IL-7 independent (LeBien 2000; Naradikian et al. 2014).
Figure 1 summarizes the process of early B cell development and Figure 2 provides an overview of the characteristics of early B cell subsets in human and mouse.

Fig. 1. Summary of early B cell development. MPPs, multipotent progenitors; CLPs, common lymphoid progenitors; BM, bone marrow; RAG, recombinase activating genes; TdT, terminal deoxynucleotidyl transferase; SLC, surrogate light chain; BCR, B cell receptor
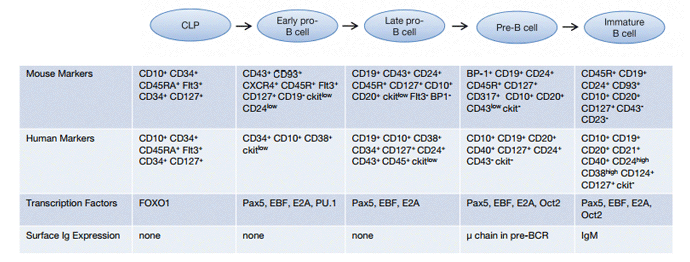
Fig. 2. Early stages of B cell development. Ig, immunoglobulin; Pax5, paired box gene 5; EBF, early B cell factor 1; OCT2, octamer transcription factor 2; forkhead box protein O1, FOXO1.
B cell maturation in the periphery
Immature B cells will exit the bone marrow within several days and enter circulation as transitional B cells. Transitional B cells are subdivided into three groups, T1, T2 and T3 B cells, based on cell surface marker expression and function (Palanichamy et al. 2009). These cells can be found in the blood and secondary lymphoid organs but rarely enter the lymphatics. These transitional cells represent the final stage before differentiation into more mature pre-immune B cell pools, namely follicular (FO), marginal zone (MZ), germinal center (GC), and memory B cells. The mechanisms that determine which mature B cell subset transitional B cells enter are not fully understood; however, cytokine availability, BCR specificity and competition with pre-existing mature B cells all play a role (Naradikian et al. 2014).
T1 B cells mature into T2 B cells based on signaling via the B lymphocyte stimulator (BLyS; also known as BAFF) family of ligands and receptors. In addition to BLyS, these include A proliferation-inducing ligand (APRIL), BLyS receptor 3 (BR3 also known as BAFF-R), transmembrane activator and cyclophilin ligand interactor (TACI) and B cell maturation antigen (BCMA) (Naradikian et al. 2014). Transitional B cell maturation occurs primarily in the spleen, with T1 B cells present in the red pulp and outer periarterial lymphatic sheath (PALS) and T2 cells in the follicles (Chung et al. 2002). Antigen-based selection of transitional B cells also occurs in the spleen to ensure the survival of mature B cells with low avidity to self-antigens and the destruction of B cells with high affinity to self-antigens. T2 B cells then differentiate into either circulating B cells that organize into GCs, or non-circulating B cells that populate the marginal zones. B cell maturation in GCs is associated with somatic hypermutation of antibody V region genes, which provides the basis for the generation of antibodies with high affinity antigen receptors (see section 3) (Tobòn et al. 2013). Antigen selected B cells that leave the GC go on to become memory B cells or plasmablasts. Table 2 highlights the cell surface markers, associated transcription factors and location of peripheral B cell subsets in mouse and human.
Table 2. Characterization of peripheral B cell subsets in mouse and human
|
Subset |
Phenotype |
Associated TFs |
Cellular Location |
|---|---|---|---|---|
|
HUMAN |
||||
|
|
Transitional |
CD20+ CD27- CD38hi IgM+ CD24hi BR3+ |
Pax5, EBF, E2A, Oct2 |
Migration from BM to secondary lymphoid organs |
|
Follicular |
IgMlo CD23+ CD93- CD19+ CD20+ CD21+ CD22+ |
Pax5 |
Shuttling between BM and secondary lymphoid organs |
|
|
Marginal zone |
IgMhi IgDlo CD1c+ CD24+ CD19+ CD20+ CD21+ |
Pax5, EBF, E2A, Oct2 |
Secondary lymphoid organ |
|
|
Germinal Center |
CD20+ CD38+ BR3+ IgD- |
BCL6, Pax5, EBF |
Secondary lymphoid organ |
|
|
Plasma cells |
CD20- CD38hi CD27hi CD138+ TACI+ and/or BCMA+ CD126+ CD319+ CD78+ |
BLIMP1, IRF4, XBP1 |
Long lived plasma cell in BM. Short lived plasma cell in secondary lymphoid organs |
|
|
Memory B Cell |
CD20+ CD38- CD27+ CD80+ CD84+ CD86+ |
OBF1, SPI-B |
Circulating in both BM and lymphoid locations |
|
|
MOUSE |
||||
|
|
Transitional |
B220+ CD93+ CD24hi IgM+ BR3+ TACI+ |
Pax5, EBF, E2A, Oct2 |
Migration from BM to secondary lymphoid organs |
|
Follicular |
IgMlo CD45Rhi CD38+ CD23+ CD22+ CD19+ |
Pax5 |
Shuttling between BM and secondary lymphoid organs |
|
|
Marginal zone |
IgMhi IgDlo CD1d+ CD9+ CD21+ CD22+ CD35+ CD45R+ CD23+ |
Pax5, EBF, E2A, Oct2 |
Secondary lymphoid organ |
|
|
Germinal Center |
CD45R+ GL7+ CD95+ PNA+ BR3+ IgD- IgM- |
BCL6, EBF |
Secondary lymphoid organ |
|
|
Plasma cells |
IgD- CD45Rlo |
BLIMP1, IRF4, XBP1 |
Long lived plasma cell in BM. Short lived plasma cell in secondary lymphoid organs |
|
|
Memory B Cell |
CD45R+ CD80+ CD73+ CD273+ CD38+ CD84+ CD86+ |
Pax5, OBF1, SPI-B |
Circulating in both BM and lymphoid locations |
|
Adapted from Naradikian et al. 2014 and Melchers 2015. TFs, transcription factors; Pax5, paired box gene 5; EBF, early B cell factor 1; OCT2, octamer transcription factor 2; BCL6, B cell lymphoma 6 protein; IRF4, interferon regulatory factor 4; XBP1, X-box binding protein 1;TACI, transmembrane activator and cyclophilin ligand interactor; BCMA, B cell maturation antigen; BR3, B lymphocyte stimulator receptor 3; Ig, immunoglobulin.
B cell activation and the humoral immune response
B cell activation is initiated when the IgD and monomeric IgM surface receptors of B cells bind to specific antigens. Upon encounter with a microbe or antigen, either by infection or vaccination, naïve B cells (antigen inexperienced) become activated and differentiate into antibody-producing plasma cells and memory B cells. Some plasma cells migrate to the bone marrow, where they persist for several years and continue to produce antibodies even in the absence of antigen.
There are two routes to B cell activation and initiation of the humoral immune response, which depend on the nature of the antigen. Non-protein antigens such as lipids, nucleic acids and glycoproteins stimulate antibody production in the absence of T cells, and are referred to as thymus independent (TI) antigens. In contrast, the antibody response to protein antigens requires both B and T cell involvement, and these antigens are described as thymus dependent (TD) antigens.
T cell independent B cell activation
TI antigens can further be subdivided into type I and type II antigens. Type I TI antigens are mitogenic stimuli such as lipopolysaccharide (LPS), CpG or poly IC. These stimuli induce B cell activation through toll-like receptors. On the other hand, type II TI antigens are generally polysaccharides that engage the BCR and thus induce antigen specific B cell responses. TI type I antigens possess an intrinsic capacity to directly induce B cell division. However, they are incapable of inducing efficient isotype switching or affinity maturation, which requires T cell help. At high concentrations, TI type I antigens induce the proliferation and differentiation of most B cells regardless of antigen specificity (polyclonal activation). However, at low concentrations, they induce antigen-specific antibody responses. Whereas TI type I antigens can activate both immature and mature B cells, TI type II antigens only activate mature B cells (Janeway et al. 2001). These antigens act by extensively cross-linking the BCRs of mature B cells specific for the antigen. However to avoid inducing B cell anergy (unresponsiveness to antigens) through intense BCR cross-linking, epitope density is critical for activation of B cells by type II TI antigens. At low density, receptor cross-linking is insufficient to induce activation, however at too high density, the B cell becomes anergic.
Days after TI challenge, substantial numbers of plasma cells accumulate in the splenic extrafollicular regions (Naradikian et al. 2014). The antibodies generated by this response are typically IgM and demonstrate low affinity for antigen compared to antibodies derived using T cell help. Within 2-3 weeks, the majority of these plasma cells die; however some persist long term and memory B cell formation is also observed (Obukhanych and Nussenzweigh 2006). Consequently, TI responses are often of shorter duration than T cell dependent B cell responses.
T cell dependent B cell activation
T cell dependent B cell activation occurs in two anatomically distinct phases. The early phase occurs in the T cell area and primary follicles and involves B cell proliferation, initial antibody secretion and isotype switching. In the late phase, affinity maturation and B cell memory formation occurs, and this takes place in GCs within lymphoid follicles. Within 1-2 days of antigen exposure, naïve CD4+ T cells become activated through recognition of antigen presented by professional antigen presenting cells in the T cell area of lymphoid organs. Simultaneously, B cells recognize the same antigen and also become activated and move from the follicle into the T cell area. The antigen activated T and B cells interact at the interface of the follicles and the T cell area, which occurs 3-7 days after antigen exposure.
T cell mediated activation of B cells is initiated when antigen specific B cells bind antigens via Ig receptors, thereby enhancing the expression of co-stimulatory molecules on the B cell surface. The B cell then internalizes the antigen bound receptor through receptor mediated endocytosis. The antigen is then processed internally and peptide fragments are presented on the cell surface via MHC Class II molecules to cognate CD4+ T cells. The B cell also expresses B7-1 and B7-2 during antigen processing. The CD4+ T cell that recognizes the MHC-peptide complex on the surface of the B cells binds B7 via CD28 (on T cells), which leads to T cell proliferation. Once activated, the T cells then express CD40L which binds to CD40 on the B cell surface. This leads to the transcription of immunoglobulin genes, the release of cytokines from T cells and B cell proliferation. The T cell secreted cytokines function to amplify B cell proliferation and differentiation as well as to determine the type of antibody produced by promoting isotype class switching (Table 3). Some of the proliferating B cells differentiate into effector antibody secreting cells, with antibodies of the same specificity as the initial antigen recognizing Ig receptor. These antibody secreting cells can be found in the extrafollicular sites of lymphoid tissue or they can migrate to the bone marrow 2-3 weeks after antigen exposure.
Cytokines mediate isotype switching following CD40-CD40L interaction. The mechanism of isotype switching is referred to as switch recombination in which the VDJ gene segment recombines with a downstream C region gene, and deleting the intervening section. The antibodies that are initially secreted are of the IgM subtype; however, isotype switching leads to the production of antibodies with heavy chains of different classes (Table 3).
The late phase of T cell dependent B cell activation involves formation of GCs. Within 4-7 days of antigen exposure, clusters of proliferating B cells can be observed at the borders of B cell follicles and T cell zones in the lymph nodes and spleen (Nieuwenhuis and Opstelten 1984; Jacob et al. 1991). These are called GCs, which are temporary structures in which the functional features of TD responses emerge, particularly affinity maturation and formation of memory B cells and long lived plasma cells. GCs contain cells derived from only one or a few antigen-specific B cell clones. In the GC, follicular dendritic cells, found only in lymphoid follicles, express complement receptors (CD35/CR1, CD21/CR2, CD11b/CD18 (CR3)), Fc receptors and CD40L. These molecules stimulate GC B cells leading to their proliferation and accumulation in the basal dark zone of the GC. Affinity maturation of B cells also occurs at this stage. This is the process of generating high affinity antigen specific antibodies as the T dependent humoral response progresses. This is mediated through somatic mutations in Ig genes followed by selective survival of B cells producing antibodies of the highest affinity. Both somatic hypermutation and isotype class switching are mediated by the gene activation-induced deaminase (AID) (Muramatsu et al. 2000).
After the completion of T dependent B cell maturation in GCs, some of the antigen activated B cell develop into memory B cells that are capable of mounting rapid antibody responses upon secondary exposure to an antigen. Figure 3 summarizes the stages involved in the secondary phase of B cell activation in GCs, and Figure 4 provides a complete schematic of B cell development and maturation from stem cells to activated B cells.
Table 3. Cytokines involved in isotype switching during T cell dependent B cell activation
Heavy chain produced |
Cytokines mediating isotype switch |
|---|---|
|
IgG1 |
IL-4 |
|
IgG2a |
IFN-γ |
|
IgG2b |
TGF-β |
|
IgG3 |
IFN-γ |
|
IgE |
IL-4 |
|
IgA |
TGF-β, IL-5 |
Adapted from Janeway et al. 2001. Ig, immunoglobulin; IFN-γ, interferon-gamma; TGF-β, transforming growth factor beta; IL, interleukin.

Fig. 3. Overview of T cell dependent B cell activation in germinal centers. Adapted from Rao S. GC, germinal center; Ig, immunoglobulin; Ag, antigen; DCs, dendritic cells; ab, antibody
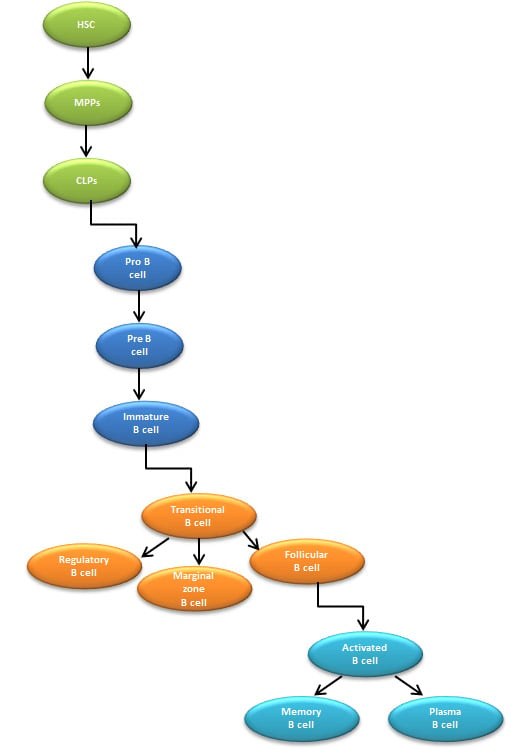
Fig. 4. Overview of B cell lineage differentiation. HSC, hematopoietic stem cells; MPPs, multipotent progenitors; CLPs, common lymphoid progenitors.
B cells as therapeutic targets
B cells have been shown to be important mediators of autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). They contribute to pathological immune responses through the production of autoantibodies, presentation of self-antigens, inappropriate co-stimulation of T cells and cytokine secretion. In addition, formation of the GC has been shown to be critical for inducing lymphomagenesis (Basso and Dalla-Favera 2015). Accordingly, about 80% of B cell non Hodgkin lymphomas are derived from GC B cells (Basso and Dalla-Favera 2015).
The increased understanding of B cell biology established over the past 20 years has led to the development of therapeutic agents for treating autoimmune diseases and lymphomas driven by aberrant B cell function. The most common therapeutic strategies include targeting B cell specific markers, depleting survival factors and disrupting intercellular or intracellular B cell functions (Naradikian et al. 2014). Below, we discuss current therapeutics that utilize these strategies for treating B cell diseases.
Targeting B cell specific markers
The most common strategy for B cell mediated disease therapy is targeting B cell specific markers for removal of aberrant B cells. The first B cell targeting therapeutic antibody approved by the US Food and Drug Administration was Rituximab, a mouse/human chimeric IgG1 monoclonal antibody. Rituximab directly depletes aberrant B cells by targeting the CD20 surface molecule. Rituximab was originally developed for the treatment of B cell malignancies but is also used to treat moderate to severe RA as well as other autoimmune diseases such as Wegener’s granulomatosis and microscopic polyangiitis (Blüml et al. 2013). Other therapeutic antibodies targeting CD20 currently in development include Ocrelizumab, Ofatumumab and Veltuzumab. An engineered protein against CD20 called TRU-015 has also been developed (Tobòn et al. 2013; Blüml et al. 2013).
The B cell surface molecule CD19 is also a therapeutic target. In contrast to CD20, CD19 is maintained on plasmablasts and subsets of plasma cells. This surface marker also regulates the threshold for B cell activation. Therefore, changes in the expression of CD19 can result in loss of tolerance and autoantibody production (Engel et al. 1995). MEDI-551 is an affinity-optimized monoclonal antibody targeting human CD19 that functions primarily by inducing antibody dependent cellular cytotoxicity (ADCC) (Herbst et al. 2010). Studies show that MEDI-551 successfully depletes B cells from blood and lymphoid organs at lower doses than Rituximab (Blüml et al. 2013).
CD22 is also targeted therapeutically via the human monoclonal antibody Epratuzimab. Similar to the anti-CD19 antibody MEDI-551, epratuzimab exerts modest ADCC. It’s predominant function however is to trigger signaling events in B cells. When immobilized, epratuzimab also interferes with anti-IgM stimulated cell proliferation (Blüml et al. 2013).
Depleting survival factors
Inhibiting the effects of the B cell survival factor BLyS on B cells is also an advanced therapeutic strategy for addressing B cell mediated diseases. This is achieved through the use of anti-BLyS or anti-BR3 monoclonal antibodies as well as BR3 or TACI decoy fusion proteins (Tobòn et al. 2013). Belimumab and tabalumab are anti-BLyS monoclonal antibodies that neutralize soluble BLyS, resulting in apoptosis of B cells. Belimumab was the first biologic therapeutic approved for treatment of SLE and tabalumab is currently being pursued for RA and SLE treatment (Blüml et al. 2013). A novel approach for targeting BLyS involves the use of a fusion protein comprised of the extracellular domain of TACI fused to the Fc region of human IgG. This therapeutic protein is called Atacicept (TACI-Ig), and it binds and blocks both BLyS and APRIL to inhibit B cell maturation (Gross et al. 2001).
Disrupting intercellular B cell function
Because the interaction of B cells with T cells is critical for inducing humoral immunity, impeding interactions of activated B cells with aspects of T cell help is a promising strategy for treating B cell diseases. Consequently, several blocking monoclonal antibodies targeting CD40L have been developed and tested in clinical trials. However, several of these antibodies such as ruplizumab and toralizumab demonstrate platelet dependent adverse effects in patients (Blüml et al. 2013). CDP7657 is an anti-CD40L Fab fragment under development for treating SLE. It is designed to counteract the adverse effects of previous CD40L blocking antibodies. The monovalent nature of the molecule and lack of the Fc region minimizes or eliminates the risk of platelet aggregation and thromboembolisms observed with anti-CD40L antibodies in the IgG1 format (Blüml et al. 2013).
Disrupting intracellular B cell function
Because plasma cells secrete large amounts of antibody, inhibition of proteasome function has been a unique approach to removing this B cell subset since it results in apoptosis via the unfolded protein response. Bortezomib is a proteasome inhibitor that was originally developed for treating multiple myeloma. Bortezomib was shown to selectively target TD generated plasma cells while sparing TI type II responses. However, this approach lacks the specificity of monoclonal antibodies due to the global molecular effect of bortezomib (Fierabracci 2012).
Although there has been significant progress in the advancement of therapies for B cell mediated diseases based on the wide knowledge of B cell biology, further research is required to fully understand the underlying genetic abnormalities that predispose individuals to these diseases (LeBien and Tedder 2008). Furthermore, studies demonstrating how B cells communicate and interact with the microenviroment will also provide greater insight into the pathophysiology of autoimmune disease. This information, combined with the results of on-going clinical trials using B cell targeted biologics will provide a basis for designing more personalized therapies with increased efficacy and minimal side effects.
References
- Alt FW et al. (1984). Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J 3, 1209-1219.
- Basso K and Dalla-Favera R (2015). Germinal centres and B cell lymphomagenesis. Nat Rev Immunol 15, 172-184.
- Blüml S et al. (2013). B-cell targeted therapeutics in clinical development. Arthritis Res Ther doi: 10.1186/ar3906.
- Burnet FM (1959). The Clonal Selection Theory of Acquired Immunity. (Nashville: Vanderbilt University Press).
- Chung JB et al. (2002). CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. International Immunology 14, 157-166.
- Cooper MD (2015). The early history of B cells. Nat Rev Immunol 15, 191-197.
- Cooper MD et al. (1965). Delineation of the thymic and bursal lymphoid systems in the chicken. Nature 205, 143-146.
- Ehrlich P (1967). Partial Cell Functions- Nobel Lecture December 11, 1908. From Nobel Lectures, Physiology or Medicine 1901-1921. Elsevier Publishing Company, Amsterdam.
- Engel P et al. (1995). Abnormal B lymphocyte development, activation and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39-50.
- Fagraeus A (1948). The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol 58, 1-13.
- Fierabracci A (2012). Proteasome inhibitors: a new perspective for treating autoimmune diseases. Curr Drug Targets 13, 1665-1675.
- Fillatreau S et al. (2002). B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3, 944-950.
- Froland S et al. (1971). Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol 234, 251-252.
- Gathings WE et al. (1977). Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in human. Eur J Immunol 7, 804-810.
- Gross JA et al. (2001). TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity 15, 289-302.
- Herbst R et al. (2010). B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther 335, 213-222.
- Jacob J et al. (1991). In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 173, 1165-1175.
- Janeway CA Jr et al. (2001). Immunobiology: The immune system in health and disease. 5th edition. (New York: Garland Science).
- Jerne NK (1955). The natural-selection theory of antibody formation. Proc Natl Sci Acad USA 41, 849-857.
- Kitamura D et al. (1992). A critical role for of lambda 5 protein in B cell development. Cell 69, 823-831.
- Komori T et al. (1993). Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science 261, 1171-1175.
- LeBien TW (2000). Fates of human B-cell precursors. Blood 96, 9-23.
- LeBien TW and Tedder TF (2008). B lymphocytes: how they develop and function. Blood 112, 1570-1580.
- Mauri C et al. (2003). Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197, 489-501.
- Melchers F (2015). Checkpoints that control B cell development. J Clin Invest 125, 2203-2210.
- Mitchell GF and Miller JF (1968). Cell to cell interaction in the immune response, II: the source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med 128, 821-837.
- Mizoguchi A et al. (2002). Chronic intestinal inflammatory condition generates IL-10 producing B cell subset characterized by CD1d upregulation. Immunity 16, 219-230.
- Muramatsu M et al. (2000). Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553-563.
- Naradikian MS et al. (2014). Understanding B cell biology. In Drugs targeting B-cells in autoimmune diseases, Milestones in drug therapy, X. Bosch et al., eds. (Springer Basel), pp. 11-35.
- Nieuwenhuis P and Opstelten D (1984). Functional anatomy of germinal centers. Am J Anat 170, 421-435.
- Nossal GJ and Lederberg J (1958). Antibody production by single cells. Nature 181, 1419-1420.
- Nunez C et al. (1996). B cells are generated throughout life in humans. J Immunol 156, 866-872.
- Obukhanych and Nussenzweigh (2006). T-independent type II immune responses generate memory B cells. J Exp Med 203, 305-310.
- Palanichamy A et al. (2009). Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182, 5982-5993.
- Raff MC et al. (1976). Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature 259, 224-226.
- Rao S. B cell activation and humoral immunity. http://www.microrao.com/micronotes/pg/humoral_immunity.pdf. Accessed June 5, 2016.
- Stashenko P et al. (1980). Characterization of a human B lymphocyte-specific antigen. J Immunol 125, 1678-1685.
- Tobòn GJ et al. (2013). B lymphocytes: Development, tolerance, and their role in autoimmunity—Focus on systemic lupus erythematosus. Autoimmune Dis 2013, 827254.
- Tonegawa S (1983). Somatic generation of antibody diversity. Nature 302, 575-581.
- von Behring E and Kitasato S (1890). Ueber das zutandekommen der diphtheria-immunitat und der tetanus-immunitat bei thieren. Deutsche Medizinsche Wochenschrift 16, 1113-1114. In Milestones in Microbiology: 1556 to 1940, translated and edited by Thomas D. Brock, ASM Press 1998, p138.
- Wei B et al. (2005). Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci USA 102, 2010-2015.
- Weigert MG et al. (1970). Variability in the lambda light chain sequences of mouse antibody. Nature 228, 1045-1047.







